Synthetic endosomes
Principal investigator: Zerial
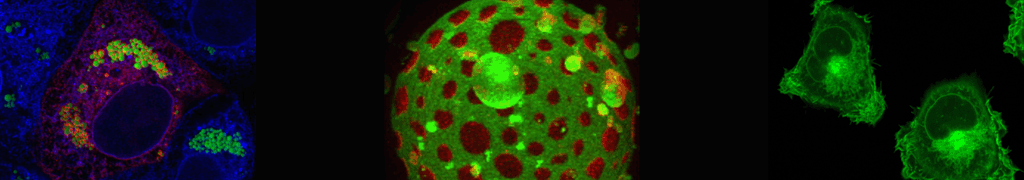
We aim to dissect the molecular mechanisms underlying early endosome tethering and fusion using in vitroreconstituted vesicles, biochemical, biophysical and microscopy-based assays. 1) We reconstituted a minimal machinery for tethering at the endosome, leading to the discovery of a novel entropic collapse mechanism whereby the endosomal tether EEA1 changes its rigidity upon binding Rab5 and brings the tethered membranes closer for fusion [Murray D. et al., Nature. 2016]. 2) We reconstituted the endosomal SNAREs and developed dynamic assays to measure membrane tethering and fusion. 3) Furthermore, we established methods (e.g. HDX-MS) to explore the structural dynamics of the Rab5 effectors, and 4) setup methods for the visualization of Rab5-domains on early endosomes in vivo (by correlative super-resolution light and electron microscopy CLEM) and their reconstitution in vitro.
Several challenging technical and instrumentation hurdles have been crossed, and we now have specific tools to discern molecular mechanisms, observe the structure of endosomal machineries, and integrate these techniques through cell biology. By visualizing structural features of the molecules, we can now discern broadly the mechanisms of tethers, scaffolds, and activating proteins in trafficking beyond endosomes. Our long-term goal is to recapitulate the function of early endosomes in synthetic endosomes. Therefore, we will address trafficking through the following specific aims:
- The molecular mechanism of entropic collapse and its generalization beyond the endosome
- The specific molecular role of Rab effectors in membrane tethering-to-fusion
- Mechanisms of spatial segregation of the Rab5 machinery on the membrane.