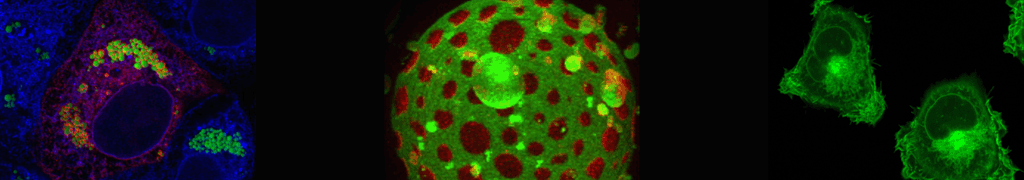
Structure-function analysis of membrane-inserted FGF2 oligomers in unconventional secretion
Principal investigator: Nickel
Fibroblast Growth Factor 2 (FGF2) is a secreted survival factor mediating tumor-induced angiogenesis. However, as opposed to the majority of extracellularly localized proteins that travel along the ER/Golgi dependent secretory pathway, FGF2 lacks a signal peptide and was shown to be secreted from cells by direct protein translocation across the plasma membrane. Several key aspects of the molecular mechanism behind this unconventional secretory pathway as well as the molecular components involved have been revealed. FGF2 secretion from cells is characterized by (i) sequential interactions of FGF2 with ATP1A1, Tec kinase and PI(4,5)P2at the inner leaflet of the plasma membrane, (ii) Tec kinase mediated tyrosine phosphorylation of FGF2, (iii) PI(4,5)P2dependent formation and membrane insertion of FGF2 oligomers and (iv) disassembly of oligomers and extracellular trapping of FGF2 mediated by cell surface heparan sulfate proteoglycans. Several lines of evidence suggest membrane-inserted FGF2 oligomers to represent key intermediates of this process forming a lipidic membrane pore with a toroidal architecture. As part of this arrangement, the headgroups of PI(4,5)P2contribute a hydrophilic surface in the periphery physically interacting with the FGF2 oligomer in the center of the membrane pore. Based upon the addition of FGF2 units at the cytoplasmic leaflet and removal of FGF2 units at the outer leaflet, an assembly/disassembly mechanism has been proposed to mediate directional transport of FGF2 across the plasma membrane. The latter process is driven by cell surface heparan sulfates that outcompete PI(4,5)P2for the interaction with FGF2 at the outer leaflet resulting in accumulation of FGF2 on cell surfaces. Recently, we have reconstituted FGF2 membrane translocation with purified components using an inside-out system based upon giant unilamellar vesicles. These studies defined PI(4,5)P2and heparan sulfates on opposing sides of the membrane along with the ability of FGF2 to oligomerize and to insert into membranes as the minimal machinery required for FGF2 membrane translocation. With the combined findings described above as a starting point, this project aims at (i) studying the role in FGF2 membrane translocation of additional components such as ATP1A1 and novel FGF2 interacting factors we have identified in a BioID screen, (ii) analyzing PI(4,5)P2dependent FGF2 oligomerization by atomistic molecular dynamics simulations and (iii) to elucidate the structure-function relationship of membrane-inserted FGF2 oligomers by correlative light and cryo-electron microscopy both in artificial membranes and in cells.